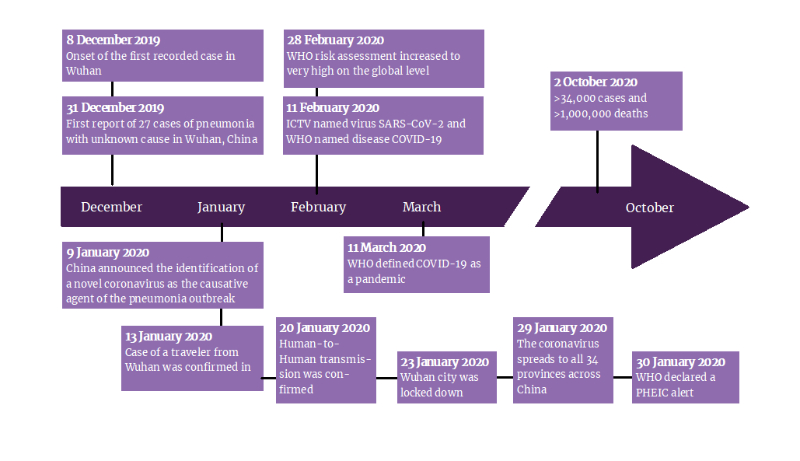
Bella Stones lived a long healthy life, traveling around the globe on a mission after retirement. As a 68-year-old American who had worked 25 years in investment banking, Bella sure had a repository of cash to finance her trips and sight-seeing missions. All was well until March 13th, 2020. Bella was stuck in China as authorities grounded all outgoing flights as an emergency directive to curb the transmission of the recently described COVID-19 infection. Bella had just 5-days on her China visit when this happened. Trapped in China, Bella continued her mission, relishing the culture and enjoying a much-needed vacation. A week later, tragedy struck.
Bella had reported to the private clinics of Metropolitan Hotel and Resort complaints of fever and generalized body weakness. Dismissing Bella's fears, the nursing assistant administered a shot of intravenous paracetamol. Four days later, Bella was admitted into the examination room of the Resort Clinic after she was found unconscious in the Hotel Lobby. Her body temperature peaked at 39.4 degrees Celsius with variable symptoms of shortness of breath, cough, expectoration, and limb weakness.
Her response to automatic coordination and reflex was also slow. Two days before presentation, she had developed diarrhea marked with about 5-6 stools per day. By coordinating with her healthcare insurance provider in Texas, the Resort Physicians established Bella's medical history of type 2 diabetes, dyslipidemia, and cirrhosis. Bella's vital signs report documented an oxygen saturation of 81%, a pulse of 100 beats/min, a respiratory rate of 27 breaths per minute, and hyperactive bowel sounds. A few hours later, Bella had also developed clinical signs suggestive of fatigue, headache, and myalgia.
Abdominal examinations revealed labored breathing sounds and audible wet murmurs in the lungs. The abdominal architecture was normal, with no lumps or pain. In line with China's guidelines on patients admitted with low oxygen saturation and high body temperature, the physician ordered a Chest CT examination considering the possibility of a COVID-19 infection. CT examination revealed bilateral pneumonia with multiple patches or ground glass appearance. Further investigations were conducted with a nucleic acid amplification test confirming the involvement of influenza A and B in Bella's illness.
A blood panel examination was ordered. The result demonstrated a considerable reduction in the normal counts of red blood cells: 2.53 × 1012 cells/l; peripheral blood hemoglobin: 72 g/l; white blood cells: 0.69 × 109 cells/l; lymphocytes: 0.22 × 109 cells/l; and platelets: 41 × 109 cells/l. The neutrophil count was elevated at 0.65 × 109 cells/L. The erythrocyte sedimentation rate and C-reactive protein level were 121 mm/hr and 71.3 mg/L, respectively.
Considering the clinical parameters obtained and Bella's history of close contact with suspected COVID-19 cases some weeks before presentation, the attending physician ordered a COVID-19 test. Blood and Saliva specimens were collected and sent to the lab. A few hours later, lab results confirmed that Bella had tested positive for the SARS-CoV-2 virus. She was immediately admitted and isolated for further treatment. In the following days on admission, Bella was administered doses of antiviral medications, including ritonavir and lopinavir. She has also been prescribed methylprednisolone sodium succinate, moxifloxacin hydrochloride sodium chloride injections, pantoprazole enteric-coated tablets, thymosin, and human immunoglobulin.
A regimen of montmorillonite powder and loperamide hydrochloride was also initiated to manage her diarrhea. Bella would eventually report a reduction in symptoms severity some days later. Her diarrhea subsided, her body temperature had dropped to 38.1 degrees Celsius, and her cough had stopped. However, repeated CT scans showed no improvement in bilateral pneumonia, and the level of blood cells showed no improvement.
Despite symptomatic improvement, Bella retested positive for the virus. As with many other cases, Bella was further started on an antiviral therapy regimen to last a few weeks before another test could be ordered.