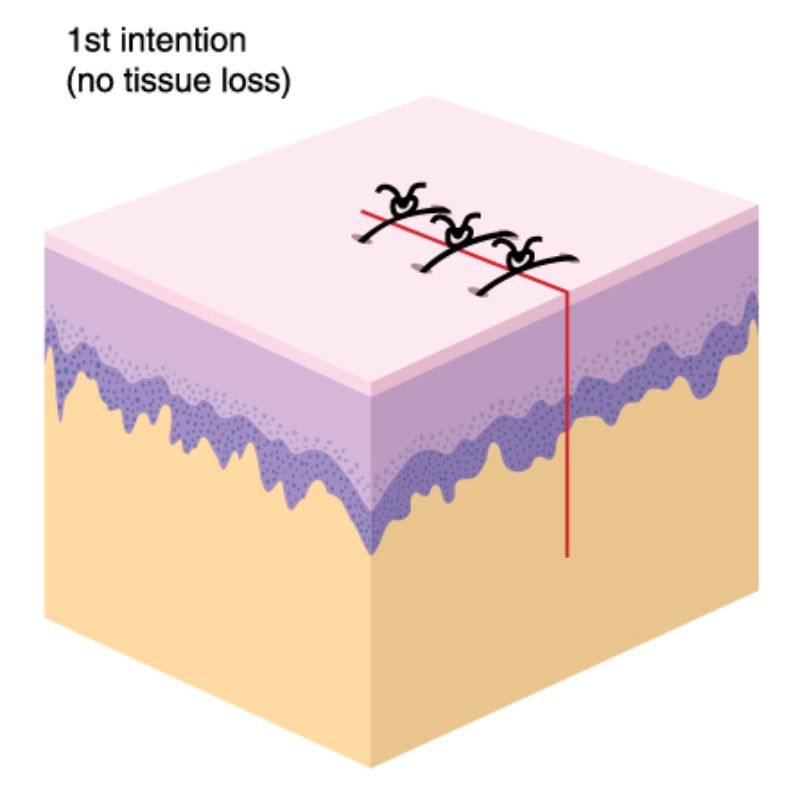
The skin covering the human body is the largest organ of the body. The skin's main role is protection (protecting the body from the environment, including chemical, radiation, microbial, and traumatic assault). However, the skin provides other essential functions with important roles in metabolism and nutrition (including fluid and electrolyte balance), thermoregulation, communication (including sensation, touch, cell signaling, emotions, etc.), and immune function. The skin has also become important for medication or therapeutic substance administration, which can be administered topically and absorbed systemically. The skin tissues receive approximately 33% of the circulating blood volume in an adult and weigh up to 15% of the total body weight of an adult (Wysocki, 2023). The skin has three main layers: the epidermis, dermis, and hypodermis (or subcutaneous tissue).
The epidermis is made up of keratinocytes or stratified squamous epithelial cells. It averages 0.075 millimeters (mm) to 0.15 mm thickness over most of the body, except on the soles and palms, where it is thicker (0.4 to 0.6 mm), and on eyelids and joints of the hands, where it is much thinner. The epidermis has five layers: stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale or stratum germinativum (basal layer). The epidermis is avascular and is constantly being renewed. The average turnover time ranges from 26 to 42 days, with complete regeneration between 45 and 75 days.
The stratum corneum (horny layer) is made up of dead keratinized cells (corneocytes) that are completely differentiated keratinocytes (80% of these cells are filled with keratin, which is insoluble and tough. The thickness of this layer varies with age, gender, and disease. The keratinocytes of this layer are mostly flat pancake-shaped cells. Wysocki (2023, p.117) notes, "Keratin is resistant to changes in temperature and to chemical digestion by trypsin and pepsin. This same protein is found in hair and nails; in these structures, keratin is referred to as 'hard' keratin compared with the 'soft' keratin of the skin." Additionally, the stratum corneum contains a lipid matrix that improves the skin's protection/barrier properties and comprises approximately 15% free fatty acids, 25% cholesterol, and 50% ceramides. This composition changes with aging.
The stratum lucidum is directly below the stratum corneum in areas where the epidermis is thicker (soles of feet and palms of hands) but is not present in areas of thinner skin (eyelids). The stratum lucidum (when present) is transparent and one to five cells thick. The actual differentiation between cells of this layer and adjacent layers can be difficult to identify under a light microscope because of the transitional nature of this layer. During transitional processes, active lysosomal enzymes degrade the nucleus and cellular organelles of keratinocytes before they are moved into the stratum corneum.
The stratum granulosum (granular layer) is one to five cells thick and lies directly beneath the stratum lucidum (when present); otherwise, it is directly beneath the stratum corneum. The stratum granulosum is named the granular layer because the keratinocytes in this layer contain keratohyalin granules. The keratinocytes of this layer are diamond-shaped because they have not yet been compressed and flattened. The cells of this layer also contain active nuclei. The keratohyalin granule structures contained in these cells are easily stained with basic dyes and an appropriate acid. Proteins in the keratohyalin granules of these keratinocytes include profilaggrin, intermediate keratin filaments, and loricrin. Loricrin is cross-linked to other protein components (cystatin A, small proline-rich proteins [SPRR1, SPRR2], elafin, envoplakin, and involucrin). Up to 70% of the molecular mass of the cells in this layer is comprised of these proteins combined.
The stratum spinosum layer is also known as the prickly layer because the keratinocytes of this layer are typically polyhedral and take on a prickly appearance due to the cytoplasmic structures in these cells. The protein involucrin is synthesized by cells in this layer, which provides a precursor of the cornified envelopes that are important in the transition of these keratinocytes into the stratum granulosum. The desmosome, a type of cell–cell junction, is one of the most prominent components of this layer. These desmosomes provide resistance to mechanical forces and increased adhesion between cells. The role and function of desmosomal proteins (plakoglobin, desmoplakin, plakophilins) and transmembrane glycoproteins (desmogleins, desmocollins) identified in this layer have been demonstrated to impact potential epidermal pathologies, such as pemphigus vulgaris, bullous impetigo, and others.
The basal layer is a single layer of basal keratinocytes, also known as basal cells. The basal layer is the main layer of the epidermis with mitotically active cells (basal cells) that respond to several factors, such as glucose, growth factors, extracellular matrix, hormones, and vitamins. After leaving the basal layer, keratinocytes begin the differentiation process. Cells leave the basal layer in an upward migration that can take 14 days to reach the stratum corneum and an additional 14 days to move through the stratum corneum to the outermost skin surface. The rete ridges at the base of the basal layer are partly responsible for anchoring the epidermis and helping ensure the skin's structural integrity. Important features of the basal layer include epidermal stem cells (which compose approximately 10% of the basal cell population), rete ridges or rete pegs, and melanocytes (approximately one melanocyte for every 36 basal cells). Wysocki (2023, p.120) states, "Dendritic melanocyte structures are responsible for the transfer of pigment to a large number of keratinocytes. The primary difference between light- and dark-skinned individuals is the size, number, and distribution of melanosomes, the structures containing the melanin pigment, and the activity of the melanocytes. Carotene or carotenoids are responsible for imparting the yellow hue to the skin of some individuals."
The basement membrane zone, BMZ, or dermal-epidermal junction, has three distinct zones: the lamina lucida, the lamina densa, and the lamina fibroreticularis. One main function of the BMZ is to anchor the epidermis to the dermis. Since no blood vessels are in the epidermis, this BMZ junction facilitates nutrient flow for metabolites and other molecules into the epithelium. This junction is affected in second-degree burns, blister formation associated with dermatologic diseases, full-thickness wounds, and mechanical trauma. With open wounds that extend deeper than the epidermis, the BMZ is disrupted and must be reformed. Wysocki (2023, p.121) states, "The major proteins found in the BMZ are fibronectin, an adhesive glycoprotein; laminin, a glycoprotein; type IV collagen, a non-fiber-forming collagen; and heparin sulfate proteoglycan, a glycosaminoglycan. A lesser amount of type VII collagen has also been detected, which forms anchoring fibrils that link the extracellular matrix of the dermis to the basement membrane."
The dermis, also known as the corium, is the thickest part of the skin: it averages 2 mm over most of the body but typically ranges from 2-4 mm in thickness. The dermis of the forehead, scalp, wrist, palm, thigh, and abdomen are thinner than the dermis of the back. Fibroblasts are the main cells found in the dermis and vary in size and number. Fibroblasts synthesize and secrete glycoproteins in the dermis, including fibronectin, thrombospondin, laminin, vitronectin, and tenascin. It has been reported that fibroblasts in darker-pigmented facial skin were larger, occurred in greater numbers, and were more likely to be binucleated or multinucleated compared with lightly pigmented skin. The implications of this related to healing have yet to be fully explored. Unlike the epidermis, the dermis is innervated (has nerve structures) and is vascular (has blood vessels). Because the dermis is vascular, it serves the body by assisting with the inflammatory response, hemostasis, thermal regulation, immune support, nutritional support, and wound management. Other cells found in the dermal layers help the skin's immune system to function properly, including lymphocytes, mast cells, and macrophages. Other structures in the layers of the dermis include sebaceous glands, eccrine and apocrine sweat glands, and hair follicles found within the dermal appendages.
The dermis consists of 2 layers: The papillary dermis and the reticular dermis. With wounds, angiogenesis (forming new blood vessels from preexisting vessels) occurs in the dermis. Angiogenesis is stimulated by vascular endothelial growth factor (VEGF), secreted by keratinocytes in response to hypoxia/skin wounding. The dermis is also largely involved in disorders involving tumor growth and metastasis, hemangiomas, telangiectasia, psoriasis, and scleroderma. Two major proteins found in the dermis are collagen and elastin. Collagen is a fiber-forming protein that accounts for 25% of the skin's total weight and gives skin its tensile strength (the ability to be stretched to a point without breaking). Collagen is primarily made up of proline, glycine, hydroxyproline, and hydroxylysine. Elastin is a fiber-forming protein, like collagen. It accounts for less than 2% of the skin's total weight and gives the skin an elastic recoil (or "bounce back" when stretched and released and prevents the skin from being permanently misshaped). Elastin has a high amount of proline and glycine but does not contain large amounts of hydroxyproline. "Ground substance" is also found in this layer, comprised of various proteins in the space between collagen and elastin fibers. Although these proteins account for only 0.2% of the dry weight of the dermis, they can bind up to 1000 times their volume. Thus, they play a role in regulating the water-binding capacity of the dermis, which helps determine dermal volume and compressibility.
The papillary dermis is immediately below the BMZ, where the papillary dermis is found. In this dermal layer, papillae (finger-like projections) form interlocking structures with the rete ridges of the epidermis. Lymphatic vessels, which help control interstitial fluid pressure by fluid resorption, are found in the papillary dermis. These lymphatic vessels also assist in clearing the tissues of cells, lipids, bacteria, proteins, and other degraded substances. The dermal papillae contain papillary loops, which supply the necessary oxygen and nutrients to the overlying epidermis via the BMZ. The papillary lymph vessels drain into larger lymph vessels in the deep subpapillary venous plexus connected to the reticular dermis lymph system. Fluid flows through this system is partially controlled through arterial pulsations, muscle contractions, and body movement.
The reticular dermis forms the base of the dermis. Cutaneous blood vessels are found in the reticular layer of the dermis. There is no clear separation of the papillary and reticular dermis layers because the size of the collagen fibers within these dermal layers changes gradually between the two layers.
The hypodermis, also known as subcutis or subcutaneous tissue, is not directly a layer of the skin but rather the subcutaneous layer below the dermis, which attaches the dermis to underlying structures.
This hypodermis or subcutaneous tissue layer helps regulate temperature, provides protection (insulation for the body, additional cushioning), and stores energy (fat). The hypodermis also assists with the mobility of the skin over underlying structures (e.g., bones and joints). The hypodermis, or superficial fascia, forms a subcutaneous layer below the dermis. It is largely comprised of adipose (fat) tissue. Sweat glands (both apocrine and eccrine) and growing hair follicles can extend into the hypodermis. This hypodermis layer also contains a reservoir of adipose-derived stem cells that help modulate skin repair and (in concert with other cells) affect paracrine and endocrine systems. This reserve supply of stem cells is decreased with aging, and their ability to respond to skin repair is reduced (Wysocki, 2023, pp. 117-124).
Clinical Pearl: The hypodermis (subcutaneous adipose tissue) is where subcutaneous injections are given via a hypodermic needle. The hypodermis is largely absent or specifically affected in certain pathologic disease states, such as Werner syndrome (also known as adult progeria), lipodystrophy, lipedema, and scleroderma. Think about how this may impact healthcare.
What considerations may be needed in these patients where the hypodermis is absent or sclerosed? - Make sure additional thermal regulation needs are met (extra blankets if hospitalized, adequate clothing, etc.).
- May need to explore alternate routes of medication administration if subcutaneous injections are ordered.
- Additional safety precautions to prevent falls and/or padding may be needed to reduce risk of bone/joint injury.
|