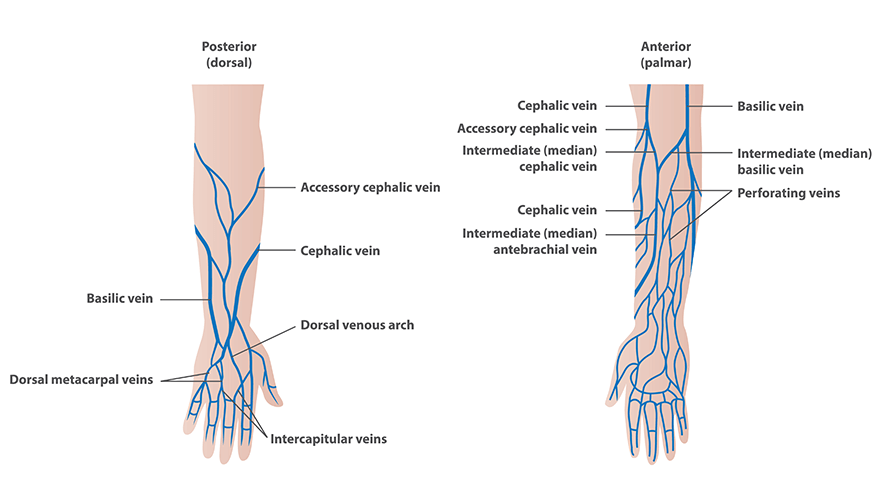
Various blood tests can be run to diagnose and manage certain illnesses. Venipuncture is performed to acquire blood for these lab tests, so they are worth reviewing (National Heart, Lung, and Blood Institute, 2022).
Antinuclear Antibody: This test measures antibodies in patients with rheumatic disease.
Blood Chemistry Studies: This test, also called a chemistry panel, measures chemicals in the body that help to show how everything is functioning. Blood chemistry studies may look at various items, such as hormones, vitamins, proteins, minerals, fats, and electrolytes, which are crucial in keeping the body functioning adequately.
Electrolyte panels are an example of a blood chemistry test. Electrolyte panels examine specific electrolytes such as magnesium, sodium, and potassium.
Renal panels are performed to look at specific functions of the kidney. Blood urea nitrogen (BUN) and creatinine are examples of tests on a renal panel.
A basic metabolic panel (BMP) looks at glucose, calcium, and the same items on a kidney function test and electrolyte panel.
A comprehensive metabolic panel (CMP) includes the same as a BMP, but also includes liver function tests.
Blood Lipid Profile: This test is also called a cholesterol panel, a lipid test/profile, or a coronary risk panel. The blood contains lipids or fat molecules. An increase or buildup of lipids in the arteries and vessels can increase a patient's risk of a heart attack or other cardiovascular complications.
There are five lipids measured in a blood lipid profile. They include:
Low-density lipoprotein (LDL): This is the bad form of cholesterol. When it builds up in the heart, it increases the chances of cardiovascular events occurring.
Very-low-density lipoprotein (VLDL): This form of cholesterol is usually low when patients are fasting as it comes from food intake. If it is high while the patient is fasting, lipid metabolism may not be functioning correctly.
High-density lipoprotein (HDL): This is the good cholesterol. It prevents the bad cholesterol from building up in the arteries and vessels.
Total cholesterol: Overall cholesterol levels that include HDL, VLDL, and LDL.
Triglycerides: This is from the intake of food. Intaking a lot of bad foods will cause an excess amount of triglycerides, increasing the risk of heart problems and an inflamed pancreas.
B-type Natriuretic Peptide (BNP): This test measures this specific hormone and provides information on the functioning of the heart. Higher levels may mean the heart is not pumping adequately and blood is not getting to the areas it should be. An elevated BNP could also mean the kidneys are not working as they should.
Complete Blood Count (CBC): This is a very common test that measures specific details about blood cells. It looks at the number of blood cells, size, and maturity. Abnormal results can mean different things. A low number of red blood cells may mean the patient is anemic. A high white blood cell count may mean the patient has an infection. Abnormal platelet results may also mean some form of infection.
Creatinine: This test evaluates how the kidneys are functioning and if there is any evidence of disease.
C-Reactive Protein (CRP): This test reflects the liver as this protein is made in the liver. If an increased amount of CRP is found in the blood, it may indicate inflammation of some kind.
Erythrocyte Sedimentation Rate (ESR): This test is also called the sed rate and helps to show any inflammation in the body. It looks at the clumping of the red blood cells and the distance these cells fall in a tube within 60 minutes. The closer the red blood cells are to the bottom of the tube, the greater the body's inflammatory response.
Hematocrit: This test measures the amount of red blood cells in the blood.
Liver Function Tests: Many liver function tests can be performed to check the enzymes in the liver for evidence of any damage or disease.
Alanine transaminase, or ALT, helps the liver use proteins for energy. When ALT levels are increased in the blood, it may indicate the liver has some damage.
Aspartate transaminase, or AST, metabolizes amino acids. Increased levels of ALT may also indicate liver damage.
Alkaline phosphatase, or ALP, assists the liver in breaking down proteins. High levels of ALP in the blood may indicate the liver is damaged or diseased.
Albumin is an important protein in the liver used to fight infections. Low levels of albumin and total protein may indicate there has been some damage to the liver.
Bilirubin is produced when red blood cells break down. If there are elevated levels of bilirubin, it could indicate liver damage or even anemia.
Rheumatoid Factor: This test specifically measures the amount of rheumatoid factor, a type of protein, that is in the blood. Increased rheumatoid factor levels are associated with autoimmune diseases. An example of a disease with a high rheumatoid factor is rheumatoid arthritis. However, high levels have also been found in healthy patients with no identifiable cause.
These are just some examples of common blood tests. There are many others out there that are used to help identify diseases and diagnose patients.