Patient
A 76-year-old African American male, Mr. Smith, presented to the emergency room at 4 pm for a fractured right hip after falling at home. No other complaints were noted. History includes type II diabetes (well-controlled with oral medication and diet), hypertension (no current medication, ran out three months ago), height 5'10", weight 150 lbs (recent weight loss of 25 lbs. over the past three months after wife recently died). Mr. Smith lives alone but has a son who lives two hours away and visits every two weeks. He has been using a walker to ambulate for the past two years since he is "not too steady" on his feet sometimes. Mr. Smith reports he sometimes dribbles urine after urinating but denies any incontinence of urine or stool. The current pain level is eight out of ten. His blood pressure is 170/98. His HbA1c level is 7.2, and his serum glucose is 125.
Risk Assessment
The Braden Scale was used by the admission nurse. Here is how she scored the patient: Sensory Perception: 4 "no impairment"; Moisture: 4 "rarely moist"; Activity: 3 "walks occasionally"; Mobility: 3 "slightly limited"; Nutrition: 3 "Adequate"; Friction & Shear: 3 "No apparent problem" – Total score: 20 (not at risk); Visual skin assessment head-to-toe reveals no redness over any boney prominence. However, a skin tear was noted on the left forearm from a fall at home.
Prevention Intervention Strategies
The patient is placed on a foam mattress (standard for the medical-surgical unit Mr. Smith was admitted to) while awaiting hip surgery. Mr. Smith cannot have anything by mouth (NPO) after midnight, and no nutrition consult has been placed yet. He is on bed rest, and an opioid was ordered for pain relief before surgery. Mr. Smith is using a urinal for voiding during the night. The patient does not want to be turned to the side during the night due to pain.
Initial Clinical Outcomes
Mr. Smith goes to surgery without additional skin assessment, and no one documents any skin warmth at the sacral area or right heel. Perianal moisture is present but not documented by transfer personnel. Mr. Smith is prepped for surgery, and the nurse conducting the perioperative skin assessment notes intact but slightly darker skin over the sacral area. The skin is also warmer to touch over the sacral area than the surrounding skin. The nurse also notes some right hip bruising (diffuse bruising over most of the right lateral hip and thigh – not particularly over trochanter boney prominence), in addition to some right heel bogginess and warmth, and the left forearm skin tear.
Staging
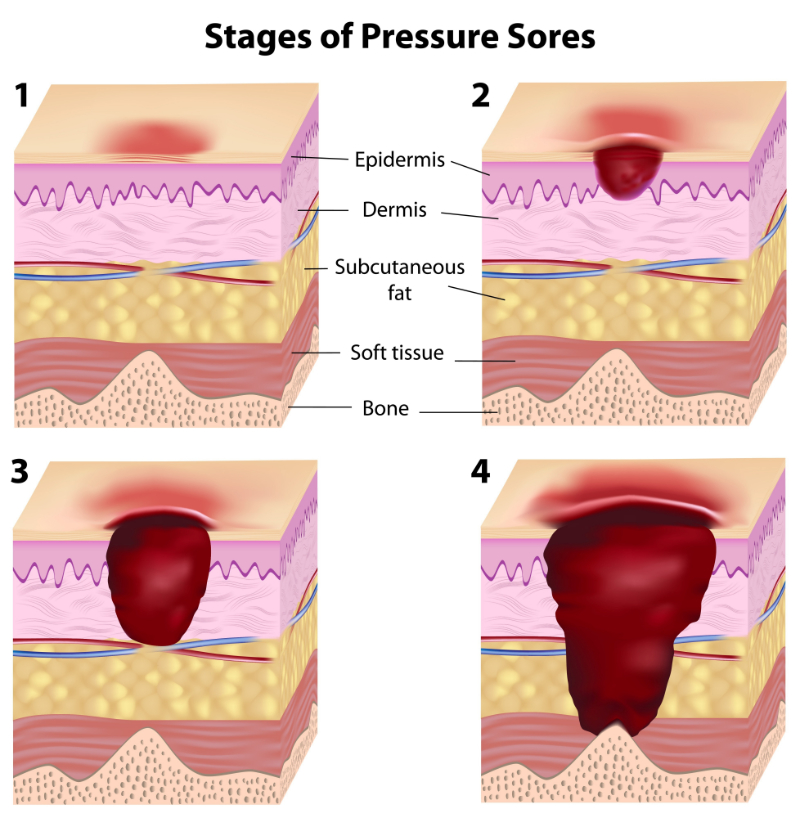
Perioperative nurse documents right hip bruising likely due to fracture injury, a stage 1 sacral PU, and stage 1 right heel PU. The left forearm skin tear is not staged because it is not a PU, and the right hip is not staged due to the nature of bruising consistent with fall injury and fracture.
Management Strategies
Perioperative staff implement specialty gel operating table padding and carefully apply foam padding to the non-operative extremities, head, etc. They also take care to float heels. In recovery, alternating pressure redistribution surface is used, small adjustments to the patient's position are made hourly to relieve pressure to the sacral area, and heels continue to be floated. Vital signs reveal high blood pressure after surgery (200/110) and elevated heart rate (110/min). Labs are unremarkable. The patient is transferred to surgical intensive care for observation. The beds have alternating pressure mattresses and beds with a "turn to assist" capability. When the patient awakes, he reports pain as four out of ten. Post-operative opioids are ordered for pain. The patient remains NPO. The perioperative staff points out all skin findings to the intensive care staff. When the patient was admitted to intensive care, the Braden risk assessment score was: Sensory Perception: 2 "very limited"; Moisture: 2 "often moist"; Activity: 1 "bedfast"; Mobility: 2 "very limited"; Nutrition: 1 "very poor"; Friction & Shear: 1 "problem" – Total score: 9 (severe risk). A nutrition consult and a wound consult by a wound specialist are ordered.
Outcomes
The patient stabilizes within one day and is transferred to a regular medical-surgical floor (on an alternating pressure specialty mattress). The staff now documents the sacral PU as stage 2 superficial open blister but no purple discoloration, and the right heel is now a deep purple color, intact, and still boggy (documented by staff as stage 1). The interdisciplinary team discusses the PU management plan and steps to initiate a care plan to prevent the progression of the existing PU and any new PU. Physical therapy initiates a mobility and strengthening care plan, and the patient starts to ambulate with assistance. A regular turning schedule is implemented at least every two hours, and sips of fluid are offered with a turning schedule. A moisture barrier cream is ordered for the perineum, and the head of the bed is kept at 30 degrees or below (when in bed) except for meals and 30 minutes after meals. Pain that may be present with turning is addressed by medicating Mr. Smith 30 minutes before the turn as needed, and nutrition interventions have been initiated. The social worker and team discuss the best location to discharge the patient, the potential need for home assistance, and the safety assessment of the home environment. Mr. Smith is discharged 12 days after the resolution of the stage 2 sacral PU with no further evolution of the heel PU. Right hip bruising is resolved, and the left forearm skin tear is closed. Mr. Smith's vital signs, including his blood pressure, pulse, and labs, are all unremarkable at discharge.
Strengths of the Case
Perioperative skin assessment was thorough and accurately noted PUs over the sacral area and right heel. The healthcare staff correctly noted right hip bruising due to injury and not a PU (diffuse bruising over most right lateral hip and thigh – not particularly over trochanter boney prominence) and the left forearm skin tear (not a PU). Perioperative staff also implement prevention strategies during surgery (specialty gel operating table padding and careful foam padding to non-operative extremities, head, etc., and take care to float heels). Alternating pressure redistribution surface is appropriately used, with small adjustments to the patient's position regularly made to relieve pressure to the sacral area, and heels continue to be floated. ICU staff correctly score the risk assessment as" 9" (very high risk):
- Sensory Perception: 2 "very limited" – because the patient is over 65 years old, and some sensory decline is normal in ages over 65 as well as people with diabetes (peripheral neuropathy present in feet), and the patient is on pain medication (decreases sensory perception, and pain may also distract the patient from feeling more minor discomforts).
- Moisture: 2 "often moist" (patient using urinal was noted to frequently spill a little, and perineal moisture was noted even pre-operatively, which could be sweat).
- Activity: 1 "bedfast" (patient was bedfast for several days).
- Mobility: 2 "very limited" (mobility was impaired severely for more than three days).
- Nutrition: 1 "very poor" (NPO for > 24 hours, 25 lb. weight loss prior to admission, and potential appetite changes due to grieving since wife recently died).
- Friction & Shear: 1 "problem" (the nurse felt the score was between a 1 or 2 because the patient was slipping down in bed frequently, needing staff to lift him back up – he was also moist in the perineal area, increasing friction, but selected 1 to capture the most risk possible, to intervene if possible).
Total score: 9 (severe risk) was accurate at admission to intensive care.
Appropriate interventions based on the level of risk considered for a Braden Scale score of 9 include: Frequent turning; maximal remobilization (early mobility, PT/OT involvement if feasible and patient's condition allows); protect heels (float heels but take care to not cause increased focused pressure to Achilles tendon – if using pillows, use one lengthwise behind each leg at calf and with enough height that heels do not touch bed surface); manage moisture (may include moisture barrier creams, avoid drying the skin, bed pads that wick moisture away from body, address cause of moisture if possible, etc.), manage nutrition and hydration (nutrition consult, increase protein intake, supplement if needed, offer liquids with turn schedules if patient is able to take oral liquids), and friction and shear (maintain head of bed below 30 degrees when not eating if condition is stable, use trapeze when indicated, use lift sheet to move patient, protect elbows and heels during movement if exposed to friction); pressure relieving support surface; turning schedule (low air loss beds or mattress overlays do not substitute for turning schedules); use of foam wedges for 30 degree lateral positioning (if medical condition allows for this); and supplementing turn schedules with small shifts in position more frequently.
The actions of the staff in the case scenario addressed some of these. Physical therapists initiated a mobility and strengthening plan of care, a regular turning schedule was implemented at least every two hours, and sips of fluid were offered with a turning schedule. A moisture barrier cream was ordered for the perineum, and the head of the bed was kept at 30 degrees or below (when in bed) except for meals and 30 minutes after meals. Pain that may be present with turning was addressed, and nutrition interventions have been initiated. Nutrition and wound consults were placed. An interdisciplinary team and social worker were involved with discharge planning, and a home safety assessment was ordered (to prevent further falls). Self-care needs at home were also evaluated/addressed.
Weaknesses of the Case
The initial Braden score obtained on admission to the hospital facility did not account for the real measure of PU risk or Mr. Smith's age as a risk factor (not accounted for by the Braden tool). The admission assessment documented:
- Sensory Perception: 4 "no impairment" (this is not accurate)
- Moisture: 4 "rarely moist" (this is not accurate)
- Activity: 3 "walks occasionally"
- Mobility: 3 "slightly limited"
- Nutrition: 3 "Adequate" (this is not accurate)
- Friction & Shear: 3 "No apparent problem" (this is not accurate)
- Total score: 20 (not at risk) was documented on admission.
However, this would have been scored more accurately if these sub-scores were marked in this way:
- Sensory Perception: 2 (between 2 "Very limited" and 3 "slightly limited," select the lower score) due to the patient's age, diabetes diagnosis, peripheral neuropathy, and pain.
- Moisture: 3 "occasionally moist" (especially at the perineum, since he did dribble urine and didn't realize it).
- Activity: 1 "Bedfast" (the patient had a newly fractured hip and would not be up walking until at least after surgery, and he was in severe pain, which impaired his mobility even in bed – so at the time of the assessment, he should have been considered bedfast).
- Mobility: 2 "very limited" (for the same reasons as activity).
- Nutrition: 1 (between 1 "very poor" and 2 "probably inadequate" for NPO status that would start after midnight and recent significant weight loss).
- Friction & Shear: 1 (between 1 "problem" and 2 "potential problem" due to the patient's severe immobility at the start of hospitalization due to hip fracture and severe pain.
The total admission score should have been between 10 and 12 (both are high-risk scores). Appropriate prevention interventions might have been initiated earlier if this level of risk had been communicated, and the PU he developed may have been avoided.
Additionally, there was room for improvement for the medical-surgical staff related to PU staging of the right heel, which was incorrectly staged as a stage 1 but was a deep purple color, intact, and still boggy (should have been staged as a deep tissue injury). Quicker implementation of powered pressure redistribution support surfaces and better attention to offloading heels pre-operatively may have helped prevent the PUs that developed (AHRQ, 2023; NPIAP, n.d.).