Meet Mr. Jamison, a 45-year-old male with a history of smoking and chronic obstructive pulmonary disease (COPD). He presents to the emergency department after a day of work with a sudden onset of sharp chest pain and difficulty breathing.
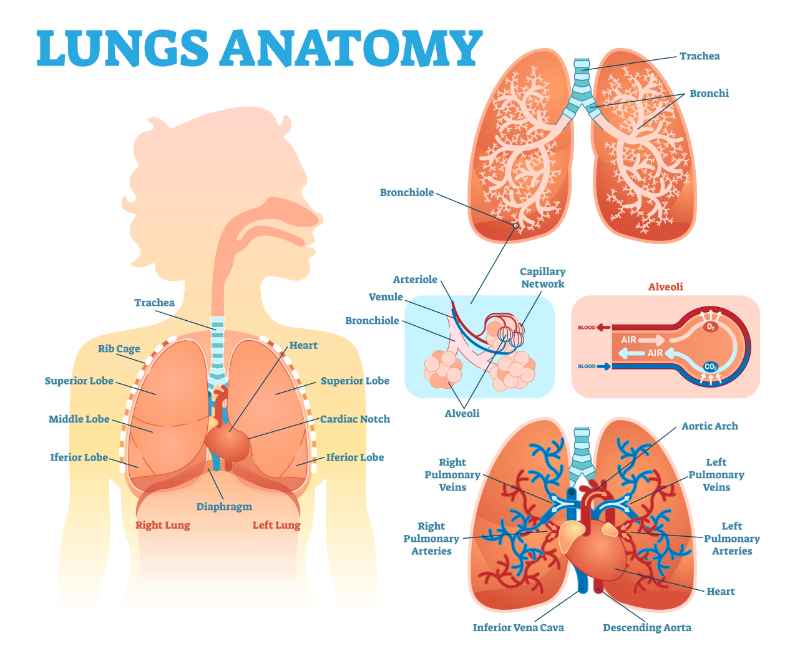
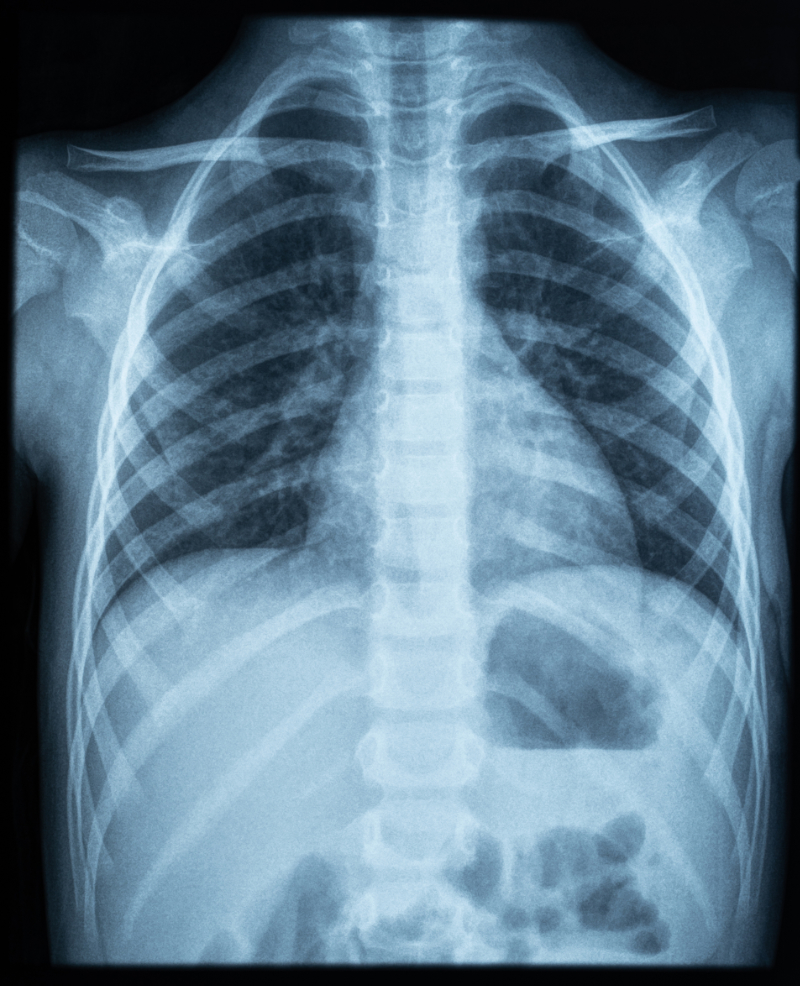
Upon assessment, Mr. Jamison appears anxious and is in respiratory distress. His respiratory rate has increased to more than twenty breaths per minute, and he has decreased breath sounds on the right side of his chest. His oxygen saturation is 88% on room air. A chest X-ray is ordered, which reveals a large right-sided pneumothorax with mediastinal shift.
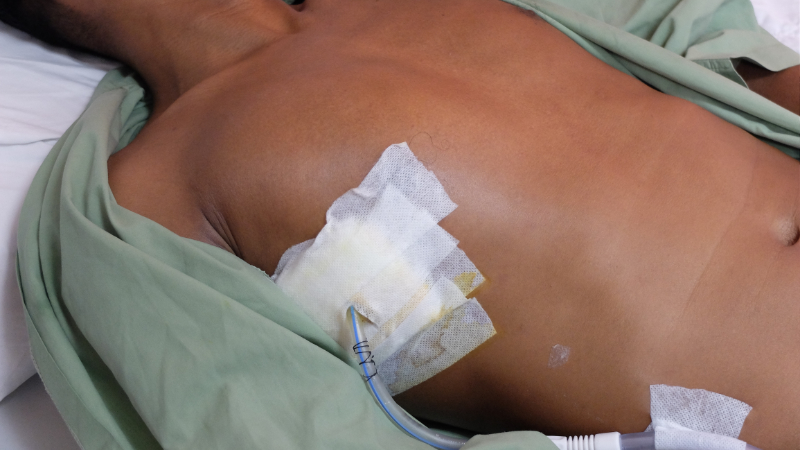
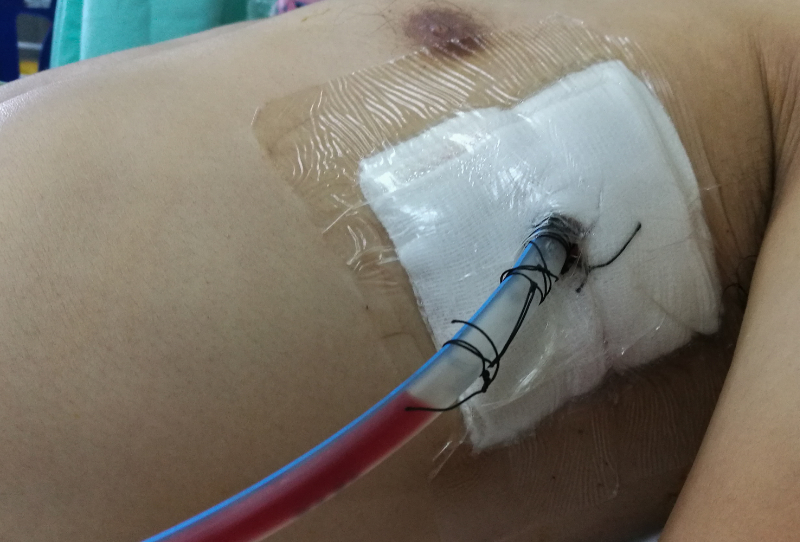
The healthcare team quickly initiates interventions to stabilize Mr. Jamison's condition. They provide supplemental oxygen via a non-rebreather mask and establish intravenous access for fluid resuscitation and pain management. A chest tube is inserted on the affected side to evacuate the air from the pleural space and re-expand the collapsed lung.
Over the next 24 hours, Mr. Jamison's respiratory distress improves, and his oxygen saturation increases to 95% on room air. The healthcare team closely monitors his vital signs, respiratory status, and chest tube drainage. They administer analgesics to manage his pain and provide education on the importance of deep breathing and early mobilization.
Mr. Jamison's chest X-ray is repeated after 48 hours, showing re-expansion of the lung and resolution of the pneumothorax. The chest tube is subsequently removed, and Mr. Jamison is discharged with instructions for follow-up care and smoking cessation support.
Had the X-ray not been completed, the pneumothorax may have been missed, leading to the patient's condition deteriorating. Because no other trauma was involved, such as in the case of a car accident or penetrating trauma, the healthcare provider can possibly take more conservative approaches, such as avoiding surgery.