Mrs. S is a 52-year-old, 5' 4", 220lbs Hispanic female with dark skin tones. Past medical history includes a history of 4 pregnancies with three vaginal deliveries, hypertension, chronic venous leg ulcers, and moderately well-controlled type II diabetes. She presents to your wound clinic with a recurrence of her right lower leg ulcer just above the medial ankle. You are the clinic wound care clinician; this is the first time you have seen this client. Mrs. S. states she is not experiencing any pain, does not smoke, rarely uses alcohol, denies using other un-prescribed substances, and complains that she is tired of this ulcer reappearing every few months. She feels self-conscious with bandages on her lower legs all the time, and they fall “a lot.”
The medical record review shows routine labs conducted three months ago were mostly within normal limits, with a hemoglobin A1c of 7.5 (a test that measures the level of hemoglobin A1c in the blood to determine the average blood sugar concentrations for the preceding two to three months). Ten months ago, she also had a normal ABI (ankle-brachial index) of 0.8 to assess her arterial perfusion.
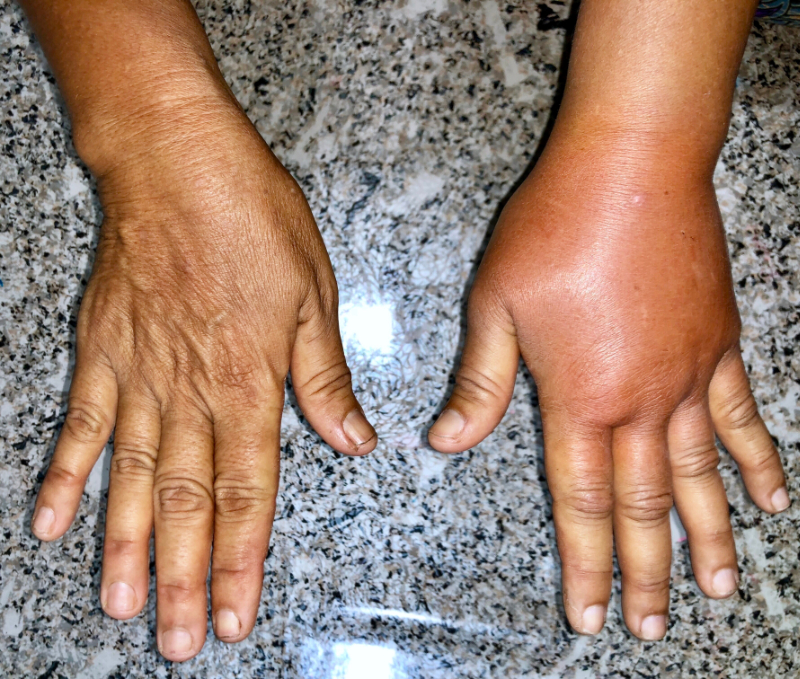
You move on to conduct a focused examination of the lower extremities. You remove ace wraps from both legs, which she says she applies daily. However, she has prescription compression stockings (30-40 mmHg, firm compression), which are about ten months old; she says “they are all stretched out” as far as they will go (she admits to putting them in the washer and dryer sometimes, more than once a week). You also remove a hydrocolloid dressing from over the right leg wound; you note there is some swelling of both lower extremities, but the right lower extremity appears slightly more edematous than the left. Her dorsalis pedis and posterior tibial pulses are fairly strong(3+) bilaterally, and you note there was 2+ pitting in the right foot, evidence when you were pressing on the dorsalis pedis pulse in the right foot (and 1+ noted in the left foot).
There is a shallow 2.5cm diameter ulcer with irregular margins located over the medial right ankle above the malleolus, which you expected to see as it is consistent with a classic venous ulcer. Mrs. S. complains that it “itches” a lot around the lower edge of the wound, especially at night. While there is slight maceration and very slight peri-wound redness extending 0.5 cm from the inferior wound edge, the bed is pink, moist, and otherwise unremarkable.
You also notice something else. The toes of the right foot look like stacked inner tubes, and when you attempt to pinch the skin at the base of the 2nd toe, you cannot do so. This positive Stemmers sign suggests a lymphedema component previously not identified in the patient's chart. You continue your exam to gather more clinical clues.
Even though Mrs. S. has been wearing her stockings, both feet and lower legs are edematous, and the skin is taut. The dorsum of the right foot appears more “puffy” than the left, giving the contour of her foot a 'box-like' appearance- another indication of lymphedema. Although it is sometimes difficult to discern hemosiderin staining of the gaiter region in people with darker skin, you palpate it and feel a woody, non-compressible quality to the tissues consistent with venous insufficiency. However, you continue palpating proximally and note that the calf areas are also firm or what is often described as 'fibrotic,' and this clue is suggestive of lymphedema. The thigh and inguinal areas are spared.
You ask Mrs. S. if she has ever heard the diagnosis of 'lymphedema,' and she says she has never heard of that before. You explain that longstanding venous insufficiency can be associated with secondary lymphedema, which can make it more difficult to manage but, once properly identified and treated, can end the cycle of re-ulceration or at least minimize recurrence.
You decide that this case could likely be managed in the clinic without referral to a lymphedema specialist since there is not one in the local area, and many will not be treated while there is still an active wound. Nevertheless, you talk to Mrs. S about a potential future referral to a lymphedema therapist as an option, should it become necessary.
Since Mrs. S’s lower extremity pulses are palpable and her ABI was 0.8, you anticipate that compression can be applied at higher levels to reach therapeutic benefits and get the edema under better control while addressing the wound. Over the next few weeks, you evaluate the compression options and, after talking to Mrs. S., decide to go with multi-layer compression (absorptive contact/base layer, covered with short stretch bandage and a more elastic top layer) on both legs, verifying the amount of compression at rest, standing and walking the first time it is applied (with a pressure monitor explicitly made for this purpose). Your goal is to reach pressures between 40 mm Hg and 60 mm Hg when walking at the strongest contraction of the calf muscle. You also evaluate Mrs. S’s compression tolerance to ensure she has no discomfort from the compression at rest and her pulses are still palpable (and toenails have good capillary refill) when the legs are elevated and at rest with the bandages intact. You also choose a more breathable, antimicrobial fiber absorptive cover dressing over the wound before applying compression bandages. You schedule twice-weekly visits in your clinic to re-evaluate her progress in the first two weeks, to re-measure her legs, order her new compression stockings every six months, and give Mrs. S. instructions to hand-wash and air-dry to maintain elasticity longer.
You encourage Mrs. S. to limit her salt intake, continue efforts with weight loss (maintaining adequate low-fat protein intake), and consider starting a walking exercise program. You proceed to monitor progress with evidence-based wound care for the open ulcer. Serial circumferential measurements of both lower extremities are taken at each visit, along with wound(s) measurements, and Mrs. S. is delighted to see the measurements steadily decrease.
Finally, the ulcer is closed, and you start the next phase of combined lymphedema/venous insufficiency treatment: maintenance. If there is a short gap of time to await new compression stockings, you realize that if you discontinue compression therapy while the custom stockings are being made, there is a good chance of the ulcer reappearing. Since no reimbursement exists for continued compression wrapping on intact legs, you place two layers of tubular compression material over each leg and instruct her to remove the second layer at night to avoid compromising the circulation and replace both layers during the day.
Mrs. S. returns to the clinic in about three weeks with her new stockings (ordered in a darker brown color to blend with the patient’s natural skin tones better). Her legs have increased slightly in circumference, but thankfully, the ulcer has not reopened. You start her on daily wear of her new stockings with instructions to apply them first thing in the morning when she wakes up, before she can walk around, and remove them at night just before retiring to bed. You reinforce washing them by hand at night, air drying them overnight for wear the next day, and schedule a return visit in 2 weeks. If possible, you may consider two pairs of stockings – the patient can alternate washing one pair and wearing the other.