SCA and SCD refer to the sudden cessation of organized cardiac electrical activity with hemodynamic collapse.
- The event is referred to as SCA (or aborted SCD) if an intervention (e.g., defibrillation, cardioversion, antiarrhythmic drug) or spontaneous reversion restores circulation.
- The event is called SCD if the patient dies. However, the use of SCD to describe both fatal and nonfatal cardiac arrest persists by convention.
SCD is the most common cause of cardiovascular death in the developed world.
- Although the risk of SCD is higher in patients with structural heart disease, as many as 10 to 15% of SCDs occur in individuals with apparently normal hearts.
- Causes of SCD with no structural heart disease include:
- Brugada syndrome
- Commotio cordis
- Early repolarization syndrome
- Familial SCD of Uncertain Cause
- Idiopathic VF (Primary Electrical Disease)
- LQTS congenital or acquired
- SQTS
- Polymorphic VT with Normal QT Interval (CPVT)
- Third Degree (Complete) AV Block
- VF Secondary to PVCs
- WPW Syndrome and Other Forms of SVT
- Survivors of SCA should undergo extensive testing to exclude drug or toxin exposure or underlying structural heart disease that may have contributed to SCA. Therapy with an ICD should generally be recommended in survivors of SCA.
- In families of victims of unexplained SCD, a general cardiology evaluation of first- and second-degree relatives can diagnose heritable disease in up to 40% of families.
The exact mechanism of collapse in an individual is often impossible to establish since, for the vast majority of patients who die suddenly, cardiac electrical activity is not being monitored at the time of their collapse. In studies, however, of patients with cardiac electrical activity monitored at the time of their event, VT or VF accounted for most episodes, with bradycardia or asystole accounting for nearly all of the remainder.
- In most patients with VT/VF, sustained ventricular arrhythmia is preceded by an increase in ventricular ectopy and the development of repetitive ventricular arrhythmia, particularly runs of nonsustained VT. In about one-third of cases, the tachyarrhythmia is initiated by an early R on T PVC. In the remaining two-thirds, the arrhythmia is initiated by a late-cycle PVC.
- There are many cardiac and noncardiac causes for sustained ventricular tachyarrhythmia resulting in SCD. Among all SCD in all age groups, the majority (65% to 70%) are related to CHD, with other structural cardiac diseases (approximately 10%), arrhythmias in the absence of structural heart disease (5% to 10%), and noncardiac causes (15% to 25%) responsible for the remaining deaths.
The risk factors for SCA are similar to those for CHD.
The approach to primary prevention of SCD varies according to a patient's clinical profile.
For the general population without known cardiac disease:
- Apart from standard screening and management of risk factors for CHD (e.g., measurement of lipids, BP, and glucose, etc.), in patients without known cardiac disease, no additional screening tests are recommended or treatment for primary prevention of SCD.
A heart-healthy lifestyle, including habitual physical activity, a heart-healthy diet, and abstinence or cessation of cigarette smoking, is recommended for the primary prevention of SCD.
Patients with known cardiac disease (e.g., prior MI, cardiomyopathy, or HF) are at an increased risk of SCA. The approach to the primary prevention of SCA in such patients includes the following:
- Standard medical therapies that lower the incidence of SCA.
- Testing for SCA risk stratification in selected subgroups.
- ICD implantation in selected patients.
The management of SCA includes acute treatment of the arrest, and for SCA survivors, a comprehensive evaluation and secondary prevention.
- The acute management of SCA involves standard cardiopulmonary resuscitation protocols.
- Initial evaluation of the survivor of SCD includes the following:
- History and physical examination
- Laboratory testing (electrolytes, blood gas, toxin screen, etc.)
- ECG
- Identification and treatment of acute reversible causes, including:
- Acute cardiac ischemia and myocardial infarction
- Antiarrhythmic drugs or other medication (e.g., QT-prolonging drugs), toxins, or illicit drug ingestion
- Electrolyte abnormalities, most notably hypokalemia, hyperkalemia, and hypomagnesemia
- HF
- Autonomic nervous system factors, especially sympathetic activation (e.g., physical or psychological stress)
- Evaluation for structural heart disease which may also include:
- Coronary angiography
- Echocardiography
- Cardiac magnetic resonance imaging (CMR)
- Evaluation for a primary electrical disease may also include:
- Electrophysiology studies
- Exercise testing
- Ambulatory ECG monitoring
- Pharmacologic challenges
- Neurologic and psychologic assessment
- In selected patients with a suspected or confirmed heritable syndrome, evaluation of family members
Secondary prevention of SCD, usually with an ICD, is appropriate for most SCA survivors.
- Because of its high success rate in terminating VT and VF rapidly, along with the results of multiple clinical trials showing improvement in survival, ICD implantation is generally considered the first-line treatment option for the secondary prevention of SCD and primary prevention in certain populations at high risk of SCD due to VT/VF. However, there are some situations in which ICD therapy is not recommended, including, but not limited to, patients with VT/VF from a completely reversible disorder and patients without a reasonable expectation of survival with an acceptable functional status for at least one year.
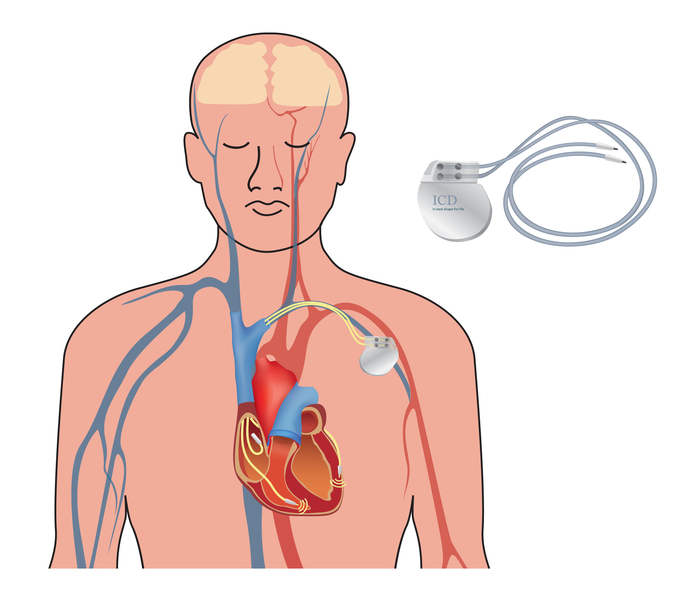
- The ICD system is comprised of pacing/sensing electrodes, defibrillation electrodes, and a pulse generator.
- Most current ICD systems utilize one, two, or three transvenous leads placed via the axillary, subclavian, or cephalic vein, with attachment to a pulse generator in the subcutaneous tissue in the infraclavicular anterior chest wall.
- DFT testing is generally performed during device implantation in patients receiving a subcutaneous ICD. It is reasonable in patients undergoing a right pectoral ICD implantation or ICD pulse generator changes (either right or left side). However, DFT testing is not required and can be omitted in patients undergoing a left pectoral transvenous ICD implantation with a right ventricular apical lead functioning appropriately.
- Contemporary ICDs have extensive storage and monitoring capacities, the ability to deliver antitachycardia pacing (i.e., overdrive pacing) to terminate VT, the ability to deliver synchronized and unsynchronized shocks for VT/VF, and the option of bradycardia pacing.
- There are a variety of complications associated with ICDs, including:
- At and around the time of implantation:
- Bleeding
- Cardiac perforation
- Infection
- Perioperative mortality
- Shoulder related problems (i.e., decreased shoulder mobility, pain, reduced function, insertion tendonitis)
- Long-term complications include:
- Lead-related problems (i.e., increased defibrillation thresholds, infection, lead failure resulting in failure to pace, failure to shock, or inappropriate shocks, tricuspid valve damage, and venous thrombosis.
- Pulse generator complications may include electronic circuit damage, electromagnetic interference, skin erosion due to the size and weight of the generator, infection of the pulse generator pocket, and Twiddlers Syndrome.
- Arrhythmia-related problems include appropriate shocks that can harm the quality of life, inappropriate shocks, usually due to the treatment of supraventricular tachycardias, and "phantom" shocks.
- Antiarrhythmic drugs can be considered the primary therapy when an ICD is not indicated or refused by the patient.
- Nearly all patients who have survived SCA should receive a beta-blocker as part of their therapy, which may also provide additional antiarrhythmic benefits.
- Because an ICD does not prevent arrhythmias, patients with symptoms or device discharges may require adjunctive antiarrhythmic therapy or consideration of catheter ablation.
- The three main indications for concomitant antiarrhythmic drug therapy are:
- To reduce the frequency of ventricular arrhythmias in patients with frequent ICD shocks.
- To suppress other arrhythmias that cause symptoms or interfere with ICD function (e.g., causing "inappropriate" shocks).
- To reduce the ventricular rate of VT so that it is better tolerated hemodynamically and more amenable to termination by anti-tachycardia pacing or low-energy cardioversion.
- For patients with an ICD who require adjunctive antiarrhythmic therapy due to ongoing arrhythmias, treatment with the combination of amiodarone plus a beta-blocker rather than treatment with amiodarone alone or other antiarrhythmic agents is recommended. This approach is especially preferred in patients with significant left ventricular dysfunction who require adjunctive antiarrhythmic therapy since amiodarone does not exacerbate HF and is less proarrhythmic than other agents.
- Adverse effects of antiarrhythmic medications include increased DFTs and slowing of the tachycardia rate, which may preclude its recognition by the ICD.
Despite advances in the treatment of heart disease, the outcome of patients experiencing SCA remains poor. The reasons for the continued poor outcomes are likely multifactorial (e.g., delayed bystander CPR, delayed defibrillation, advanced age, decreased proportion presenting with VF.)
- When SCA is due to a ventricular tachyarrhythmia, the outcome of resuscitation is better compared with those with asystole or pulseless electrical activity.
- Among the many factors that appear to influence the outcome of SCA, the elapsed time before effective resuscitation (i.e., the establishment of an effective pulse) appears to be the most critical element. There are several ways to decrease the time to the onset of resuscitative efforts:
- Rapid EMS response
- Optimizing the EMS system within a community to reduce the response interval to eight minutes or less where possible.
- Bystander CPR
- The administration of bystander CPR is an important factor in determining patient outcome after out-of-hospital SCA, as early restoration or improvement in circulation has resulted in greater survival and better neurologic function among survivors.
- Bystander CPR, however, is not always performed, primarily due to the bystander’s lack of CPR training or concerns about possible transmission of disease while performing rescue breathing.
- Early defibrillation
- The standard of care for resuscitation of SCA has been defibrillation as soon as possible when indicated.
- Shorter defibrillation response intervals correlate with greater survival to hospital discharge.
- Automated external defibrillators
- The use of AEDs by early responders is another approach to more rapid resuscitation.
- In most but not all studies, AEDs have improved survival after out-of-hospital cardiac arrest.
- Several observational studies evaluating compression-only CPR versus standard CPR, including rescue breathing, reported no significant differences in survival or long-term neurologic function between the two groups, suggesting that compression-only CPR could be safely delivered (as long as the arrest is not a respiratory arrest).
- It is recommended that if a sole bystander is present or multiple bystanders are reluctant to perform mouth-to-mouth ventilation, CPR performance using chest compressions should only be performed.
- The induction of mild to moderate hypothermia (target temperature of 32 to 34ºC for 24 hours) may be beneficial in patients successfully resuscitated after a cardiac arrest. Improved neurologic outcome and reduced mortality have been demonstrated in a series of patients with VF arrest in whom spontaneous circulation was restored, even when the patient remains comatose after resuscitation.