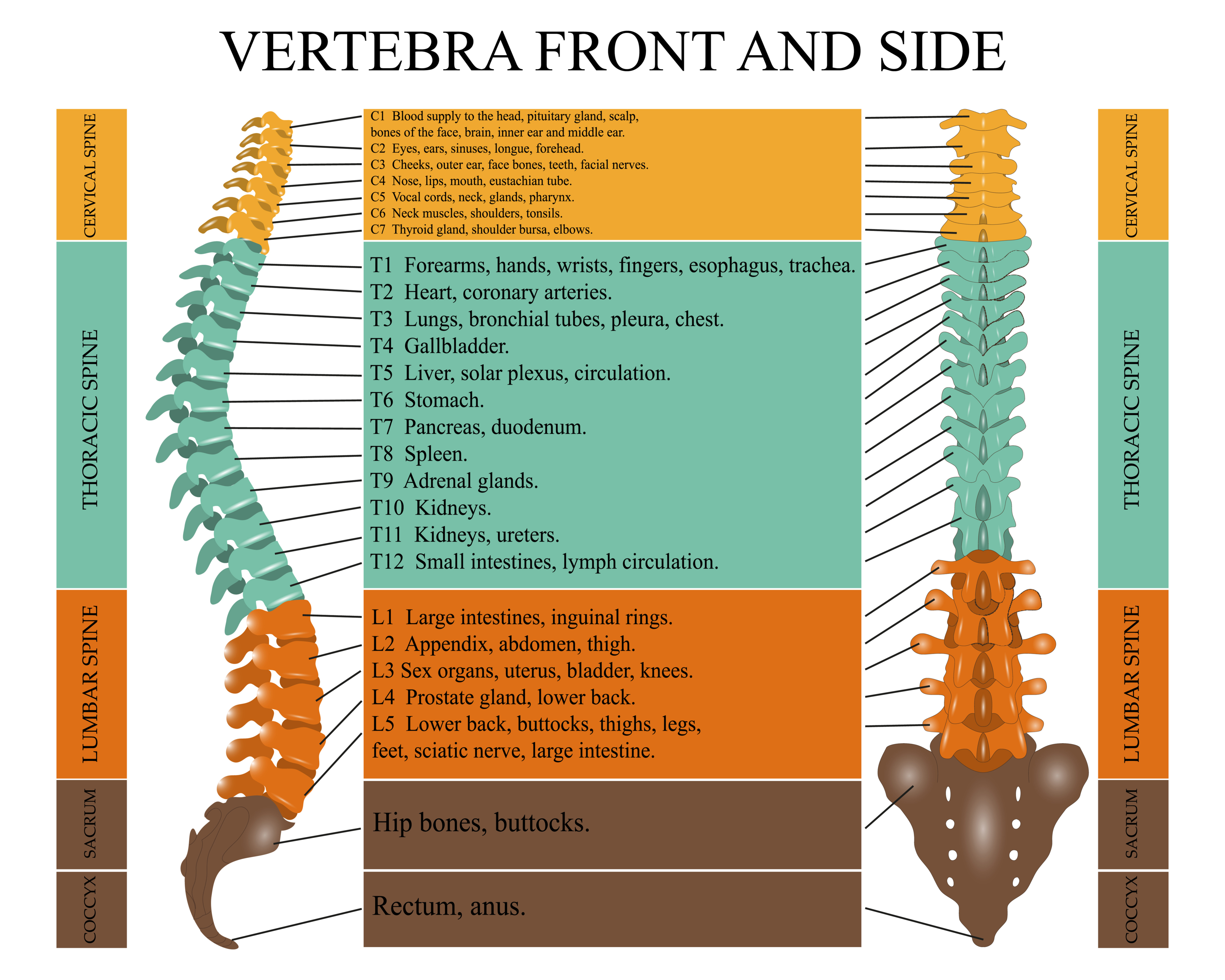
Pulmonary physiologic changes due to SCI are related to the extent of neurological impairment. The American Spinal Injury Association Impairment Scale (AIS) is used to classify the degree of impairment that is based on strength in key muscles and a sensory examination.
Immediately after SCI, flaccid paralysis affects all muscles caudal to the level of injury (i.e., spinal shock). Subsequent improvements in pulmonary function are due primarily to functional descent of the neurologic injury level as spinal cord inflammation resolves, enhanced recruitment of accessory ventilatory muscles, retraining of deconditioned muscles, and the evolution from flaccid to spastic paralysis.
The extent of ventilatory muscle impairment depends upon the degree and location of the injury, as well as the duration of time since the injury. The higher the level and more complete the injury, the more likely it is that there will be respiratory muscle dysfunction. Complete injury above C3 produces near-total ventilatory muscle paralysis because the phrenic nerve, which innervates the diaphragm, arises from the third to fifth cervical roots.
Immediately after an SCI, flaccid paralysis can occur, affecting all the muscles that are caudal to the level of injury. This physiologic state is known as spinal shock or spinal paralysis. This state may continue for days or months, and during this time, pulmonary function is impaired. This is primarily due to impairment of the muscles of respiration but also due to a decrease in chest compliance, decreased airflow, hyper-responsiveness of the bronchial passages, and an abnormally blunted response to hypercapnia.
After the resolution of spinal paralysis, spastic paralysis occurs. During spastic paralysis, muscle tone is increased, and pulmonary function should improve. This happens several days or several weeks after the injury. Improvement in respiratory muscle performance after SCI largely occurs in the first year following injury.
Assessment of pulmonary function in SCI patients is done with PFTs. Performing PFTs and calculations of the predicted PFT values need to be adjusted/modified when doing PFTs in patients who have had an SCI. Many SCI patients will have abnormal PFTs, but there is an improvement over the first few months-year after the injury.
After an SCI, the long-term course of pulmonary function can be a decline, stability, or improvement.
Approximately 90% of patients who have had a traumatic SCI will need intubation, and up to 40% of patients who had a complete cervical lesion will become ventilator-dependent. It is best to proceed with intubation under controlled circumstances rather than waiting until the condition becomes an emergency, as hypoxia in an SCI patient can adversely affect the patient’s neurological and many patients who have cervical spinal cord injury have a delayed onset of respiratory complications. Intubation is necessary for managing secretions, and SCI is often accompanied by many complications like trauma, coma, ARDS, and multi-organ system insufficiency. In these situations, non-invasive ventilation will not be effective. There are no specific criteria for ventilator settings that are specific to SCI patients.
If it is likely that the patient may be ventilator-dependent for a long time, a tracheostomy is recommended. Tracheostomy can make weaning easier by decreasing airway resistance, it can make suctioning easier and more effective, it reduces the complications of long-term endotracheal intubation, and it is more comfortable than endotracheal intubation. Tracheostomy can be done at the bedside (PT) or in the OR.
In regard to criteria for weaning eligibility, Garshick (2020) wrote: “The accuracy of weaning predictors (e.g., rapid shallow breathing index) in predicting weaning success has not been specifically addressed in this patient population, and we typically follow standard clinical criteria for weaning . . .” Schreiber et al. (2021) indicates that the probability of weaning success continues to be difficult to predict as there are no previous systematic review or meta-analysis has been conducted on the topic. Schreiber et al. (2021) also state that there are no societal guidelines or recommendations on weaning that are available and specific to this population of patients. Therefore, the typical weaning protocol is utilized. This protocol is alternating periods of exercise (the patient is disconnected from the ventilator) and rest (the patient is reconnected to the ventilator).
Conservative methods of preventing respiratory complications in SCI patients include:
- Postural drainage
- Assisted coughing
- Glossopharyngeal breathing
- Percussion and vibration
- Airway suctioning
- RMT
Phrenic or diaphragmatic pacemakers are a form of respiratory support that may be used for the partial or complete withdrawal of mechanical ventilation in patients with SCI. Electrostimulation of the phrenic nerve consists of triggering diaphragmatic contractions through direct electrical stimulation of the phrenic nerve in the neck and chest.
Non-invasive ventilation (NIV) is defined as the delivery of positive pressure ventilation without the use of an invasive device and by way of a noninvasive device like a face mask, a nasal mask, or nasal prongs. Non-invasive ventilation can be delivered by a standard or portable ventilator. The two commonly used modes of delivery are bilevel NIV, commonly called bilevel positive airway pressure (BPAP – not BiPAP), and continuous positive airway pressure, CPCP. Non-invasive ventilation is a well-established technique that can be used to treat patients who are not intubated or do not have a tracheostomy. It can also be used for those who have acute or chronic respiratory dysfunction caused by a neuromuscular or chest wall disease, including SCI. In addition, it can also be used to wean SCI patients who are endotracheally intubated, those who have a tracheostomy and are ventilator-dependent, as well as for those requiring tracheal decannulation.