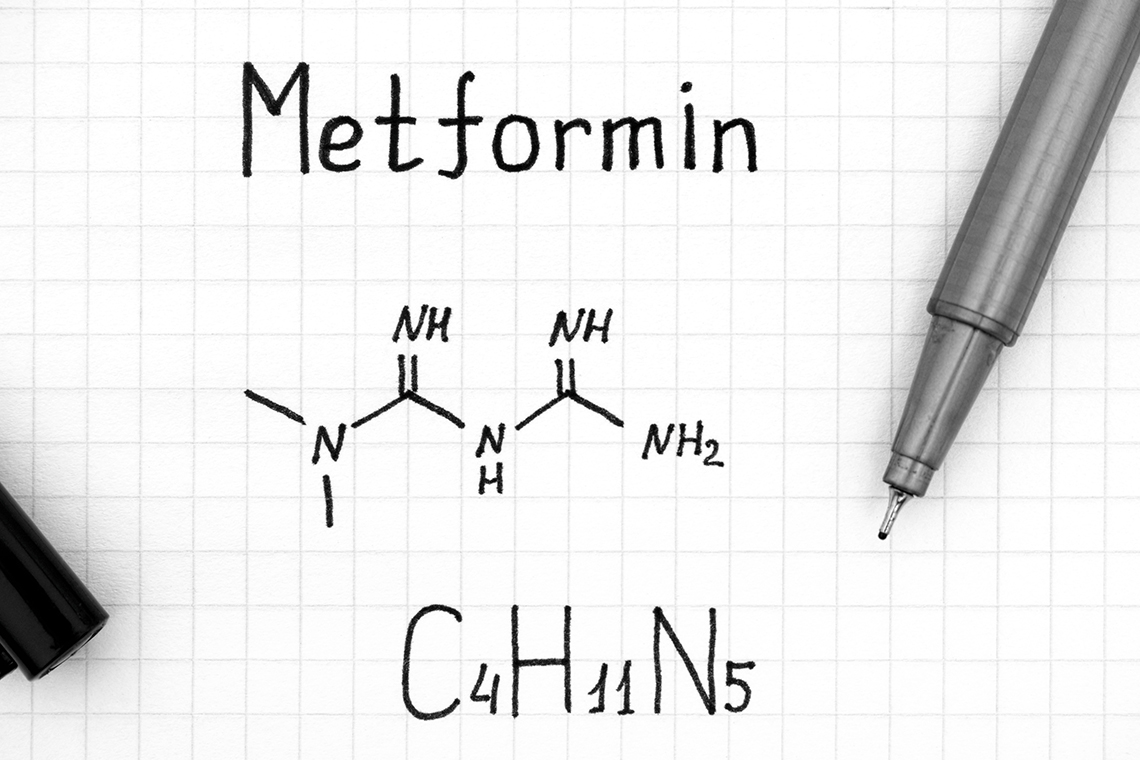
Metformin has a black box warning of lactic acidosis. Though rare, metformin can cause lactic acidosis, which has a 50% mortality rate. The risk increases if the patient has renal impairment, is older, and has unstable or acute heart failure.
Metformin is a biguanide that works by decreasing the production of glucose from the liver. Moreover, it decreases glucose absorption in the intestines. It improves insulin sensitivity to its receptors, providing a therapeutic effect of improved glycemic control with stabilized or decreased body weight and improved lipid profile.
When given orally, metformin is slowly and incompletely absorbed. Its absorption is delayed with food. It has almost negligible protein binding and is primarily distributed to the intestinal mucosa and salivary glands. It is excreted unchanged in the urine. Metformin has a half-life of 9 to 17 hours and can be removed through hemodialysis.
Metformin should be used with caution in patients at risk of developing lactic acidosis, such as those with hypoxemia, hypoperfusion, dehydration, and sepsis. Moreover, its use is contraindicated in patients with known metformin hypersensitivity, severe renal dysfunction, and acute or metabolic acidosis.
Metformin should be used with extreme caution in patients with heart failure, liver dysfunction, excessive alcohol consumption, and older adults. Its use should be temporarily discontinued if the patient has to undergo iodinated contrast imaging procedures with creatinine clearance of 30 to 60 milliliters per minute (ml/min) or with a history of heart failure and hepatic disease.
Drug Interactions
Metformin, when taken with alcohol, can increase its adverse effects. Drugs such as ranolazine, cimetidine, and dolutegravir can increase the effect or concentration of metformin when given together.
Intravenous (IV) contrast dye with metformin can increase the risk of metformin-induced lactic acidosis and renal failure. Therefore, discontinue metformin 24 to 48 hours before the procedure and do not give it until 72 hours after exposure to the contrast.
Availability
Metformin is available in tablet forms and oral solutions. The oral solution, with the brand name Riomet, is available in 100 milligrams (mg)/5 ml and tablets in 500 mg, 850 mg, and 1000 mg. Metformin is also available as extended-release tablets in 500 mg, 750 mg, and 1000 mg. Make sure not to crush or break the extended-release tablet before administration. Also, give it with food to avoid gastrointestinal upset.
Dosage
The dose of metformin given for managing type II diabetes mellitus in the form of immediate-release tablets and solutions in adults is initially 500 mg twice daily or 850 mg once a day. To titrate the metformin dose, increase by 500 mg weekly or 850 mg every other week. You can also titrate it from 500 mg twice daily to 850 mg twice daily in two weeks. The maintenance dose of metformin is 1000 mg to 2550 mg in a day, given in two or three divided doses. The maximum dose of metformin in adults that can be given daily is 2550 mg/day.
The dose of metformin for the management of diabetes mellitus in children aged 10 to 16 is initially 500 mg twice a day. If you want to titrate the dose, you can do that in increments of 500 mg. The maximum dose of metformin in children is 2000 mg, which can be given in divided doses.
For extended-release tablets of metformin given to adults, the initial dose is 500 to 1000 mg once daily. However, that can be increased by 500 mg at the one-week interval of drug therapy. The maximum dose of metformin in extended-release tablets is 2000 mg per day.
Metformin is contraindicated in patients with serum creatinine greater than 1.5 mg/deciliter (dL) in males and 1.4 mg/dL in females. It can be given to patients with a creatinine clearance of 45 to 60 ml/min, with renal function monitored every three to six months. If the creatinine clearance falls between 30 and 44 ml/min, give metformin with extreme caution and consider reducing its dose. However, with creatinine clearance lower than 30 ml/min, metformin use should be discontinued.
In patients with hepatic impairment, the use of metformin should be avoided because it can increase the risk of lactic acidosis.
Side Effects
Metformin is generally well tolerated. Rarely, it can cause side effects, such as gastrointestinal upset, causing diarrhea, nausea, vomiting, and bloating. To avoid that, metformin should be given with food. Sometimes, it can also cause a metallic taste in the mouth that resolves quickly after initiating therapy.
Adverse Effects
The adverse effects of metformin occur when it is given in more than the recommended doses. This can cause lactic acidosis, a serious condition characterized by an increase in serum lactate levels greater than 5 millimoles per liter (mmol/L). It also causes a decrease in blood pH and electrolyte imbalance, leading to symptoms such as myalgia, unexplained hyperventilation, drowsiness, and malaise that may advance to shock, acute myocardial infarction, and prerenal azotemia.
Nursing Considerations
Baseline Assessment
When initiating metformin therapy, verify that the patient has not received IV contrast dye within the last 48 hours. Also, a complete blood count, renal function tests, fasting blood glucose, and glycated hemoglobin (HbA1c) should be obtained.
Nursing Intervention and Evaluation
With metformin therapy, monitor serum glucose levels, HbA1c, renal functions, and complete blood count. Moreover, folic acid and renal function tests should be checked for evidence of lactic acidosis. If the patient receives sulfonylureas concomitantly, check them for hypoglycemia. The symptoms of hypoglycemia include cool and wet skin, increased sweating, anxiety, dizziness, headache, hunger, increased heart rate, and diplopia.
Be alert if the patient is experiencing other health conditions, such as fever, too much stress, increased activity, or surgical procedures that can affect serum glucose levels. If the patient develops lactic acidosis, withhold metformin therapy promptly.
Inform patients not to skip meals on metformin therapy as it can lead to potent hypoglycemia.
The Bottom Line
Metformin is the most commonly used oral hypoglycemic agent for the management of type II diabetes. Educate patients on indications, dosages, and adverse effects. Ensure that patients know they should report to the healthcare provider soon if they have any symptoms of lactic acidosis.
About the Author:
Mariya Rizwan is an experienced pharmacist who has been working as a medical writer for four years. Her passion lies in crafting articles on topics ranging from Pharmacology, General Medicine, Pathology to Pharmacognosy.
Mariya is an independent contributor to CEUfast's Nursing Blog Program. Please note that the views, thoughts, and opinions expressed in this blog post are solely of the independent contributor and do not necessarily represent those of CEUfast. This blog post is not medical advice. Always consult with your personal healthcare provider for any health-related questions or concerns.
If you want to learn more about CEUfasts Nursing Blog Program or would like to submit a blog post for consideration, please visit https://ceufast.com/blog/submissions.